|
Air Pollution
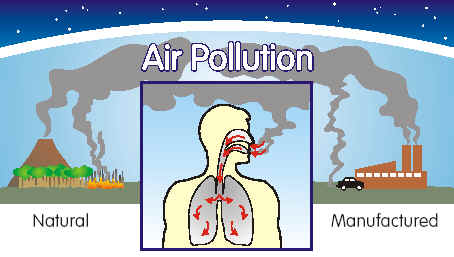
Air is the ocean we breathe. Air
supplies us with oxygen which is essential for our bodies to live. Air
is 99.9% nitrogen, oxygen, water vapor and inert gases. Human activities can
release substances into the air, some of which can cause problems for humans,
plants, and animals.
There are several main types
of pollution and well-known effects of pollution which are commonly
discussed. These include smog, acid rain, the greenhouse effect, and "holes" in
the ozone layer. Each of these problems has serious implications for our health
and well-being as well as for the whole environment.
One type of air pollution is the
release of particles into the air from burning fuel for energy.
Diesel smoke is a good example of this particulate matter . The
particles are very small pieces of matter measuring about 2.5 microns or about
.0001 inches. This type of pollution is sometimes referred to as "black carbon"
pollution. The exhaust from burning fuels in automobiles, homes, and industries
is a major source of pollution in the air. Some authorities believe that even
the burning of wood and charcoal in fireplaces and barbeques can release
significant quanitites of soot into the air.
Another type of pollution is the
release of noxious gases, such as sulfur dioxide, carbon
monoxide, nitrogen oxides, and chemical vapors. These can take part in further
chemical reactions once they are in the atmosphere, forming smog and acid rain.
Pollution also needs to be
considered inside our homes, offices, and schools. Some of these
pollutants can be created by indoor activities such as smoking and cooking. In
the United States, we spend about 80-90% of our time inside buildings, and so
our exposure to harmful indoor pollutants can be serious. It is therefore
important to consider both indoor and outdoor air pollution.
Air pollution affects everyone. Every day, the average adult breathes over 3,000
gallons of air.
The major types of air pollution
are:
Gaseous pollutants:
A different mix of vapors and gaseous air pollutants is found in outdoor and
indoor environments. The most common gaseous pollutants are carbon dioxide,
carbon monoxide, hydrocarbons, nitrogen oxides, sulfur oxides and ozone. A
number of sources produce these chemical compounds but the major man-made source
is the burning of fossil fuel. Indoor air pollution is caused by cigarette
smoking, the use of certain construction materials, cleaning products, and home
furnishings. Outdoor gaseous pollutants come from volcanoes, fires, and
industry, and in some areas may be substantial. The most commonly recognized
type of air pollution is smog. Smog generally refers to a condition caused by
the action of sunlight on exhaust gases from motor vehicles and factories.
The Greenhouse effect
prevents the sun's heat from rising out of the atmosphere and flowing back into
space. This warms the earth's surface causing the green house effect. While a
certain amount of green house gases in the atmosphere are necessary to make the
earth warm, activities such as the burning of fossil fuels are creating a
gaseous layer that is too dense to allow the heat to escape. Many scientists
believe this is causing global warming. Other gases contributing to the problem
include cholrofluorocarbons (CFC), methane, nitrous oxides, and ozone.
Acid rain forms
when moisture in the air interacts with nitrogen oxide and sulfur dioxide
released by factories, power plants, and motor vehicles that burn coal or oil.
This interaction of gases with water vapor forms sulfuric acid and nitric acids.
Eventually these chemicals fall to earth as precipitation, or acid rain. Acid
rain pollutants may travel long distances, with winds carrying them thousands of
miles before they fall as dew, drizzle, fog, snow or rain.
Damage to the ozone layer
is primarily caused by the use of chloroflurocarbons (CFCs). Ozone is a
form of oxygen found in the earth's upper atmosphere. The thin layer of ozone
molecules in the atmosphere absorb some of the sun's ultraviolet (UV) rays
before it reaches the earth's surface, making life on earth possible. The
depletion of ozone is causing higher levels of UV radiation on earth,
endangering both plants and animals.
Particulate matter
is the general term used for a mixture of solid particles and liquid droplets
found in the air. Some particles are large or dark enough to be seen as soot or
smoke. Others are so small they can be detected only with an electron
microscope. When particulate matter is breathed in, it can irritate and damage
the lungs causing breathing problems. Fine particles are easily inhaled deeply
into the lungs where they can be absorbed into the blood stream or remain
embedded for long periods of time.
Climatic effects:
Normally pollutants rise or flow away from their sources without building up to
unsafe levels. Wind patterns, clouds, rain, and temperature can affect how
quickly pollutants move away from an area. Weather patterns that can trap air
pollution in valleys or move it across the globe may be able to damage pristine
environments far from the original sources.
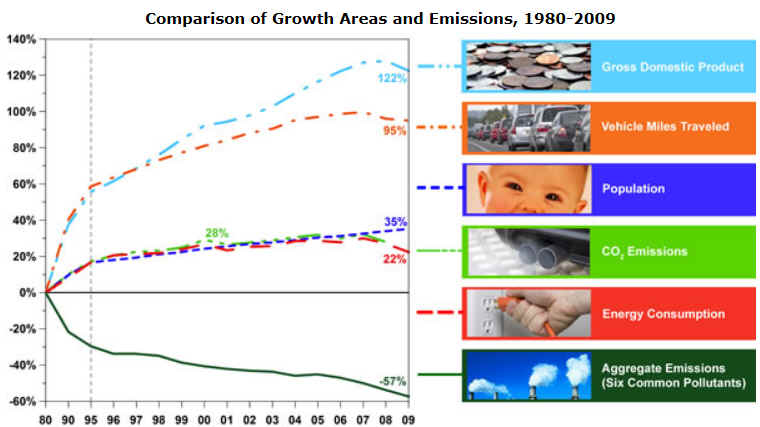
Above image for United States
Children breathe even more air per pound of body weight and are thus more
susceptible to air pollution. Millions of people live in areas where urban smog,
very small particles, and toxic pollutants pose serious health concerns. These
health concerns can stem from either short-term or long-term exposure to air
pollution. When people have a short-term exposure to air pollutants above
certain levels, they may experience temporary health concerns, such as eye
irritation and burning, throat irritation, and difficulty breathing. Long-term
exposure to air pollution can cause chronic health concerns, such as cancer and
damage to the body's immune, neurological, reproductive, and respiratory
systems. The problem of air pollution is also found outside of major urban
centers. Air pollution can be wide-ranging as well as persistent. Many air
pollutants, such as those that form urban smog and toxic compounds, remain in
the environment for long periods of time. These air pollutants can also be
carried hundreds of miles by winds and can thus affect areas far-removed from
the source of the pollution.
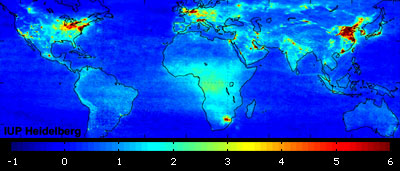
Global air pollution map produced by Envisat's SCIAMACHY
Children are very sensitive to the effects of air pollution. Children's
lungs are still developing and polluted air may contribute to
permanent lung damage. Children breathe more rapidly than do adults, and
inhale more pollution per pound of body weight than adults. Therefore, their
lungs have a greater chance for being exposed to harmful air pollutants.
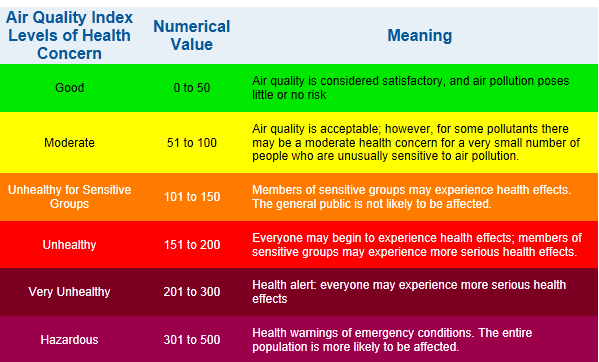
EPA Air Quality Index

The AQI is an index for reporting daily air quality. It tells you how clean
or polluted your air is, and what associated health effects might be a
concern for you. The AQI focuses on health effects you may experience within
a few hours or days after breathing polluted air. EPA calculates the AQI for
five major air pollutants regulated by the Clean Air Act: ground-level
ozone, particle pollution (also known as particulate matter), carbon
monoxide, sulfur dioxide, and nitrogen dioxide. For each of these
pollutants, EPA has established national air quality standards to protect
public health .Ground-level ozone and airborne particles are the two
pollutants that pose the greatest threat to human health in The United
States.
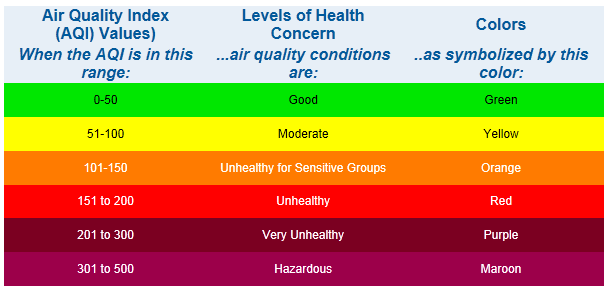
Each category
corresponds to a different level of health concern. The six levels of
health concern and what they mean are:
-
"Good" AQI
is 0 - 50. Air quality is considered satisfactory, and air pollution
poses little or no risk.
-
"Moderate"
AQI is 51 - 100. Air quality is acceptable; however, for some
pollutants there may be a moderate health concern for a very small
number of people. For example, people who are unusually sensitive to
ozone may experience respiratory symptoms.
-
"Unhealthy
for Sensitive Groups" AQI is 101 - 150. Although general public is not
likely to be affected at this AQI range, people with lung disease,
older adults and children are at a greater risk from exposure to
ozone, whereas persons with heart and lung disease, older adults and
children are at greater risk from the presence of particles in the
air. .
-
"Unhealthy" AQI is 151 - 200. Everyone may begin to experience some
adverse health effects, and members of the sensitive groups may
experience more serious effects. .
-
"Very
Unhealthy" AQI is 201 - 300. This would trigger a health alert
signifying that everyone may experience more serious health effects.
-
"Hazardous" AQI greater than 300. This would trigger a health warnings
of emergency conditions. The entire population is more likely to be
affected.
AQI colors
EPA has assigned a specific
color to each AQI category to make it easier for people to understand
quickly whether air pollution is reaching unhealthy levels in their
communities. For example, the color orange means that conditions are
"unhealthy for sensitive groups," while red means that conditions may be
"unhealthy for everyone," and so on.
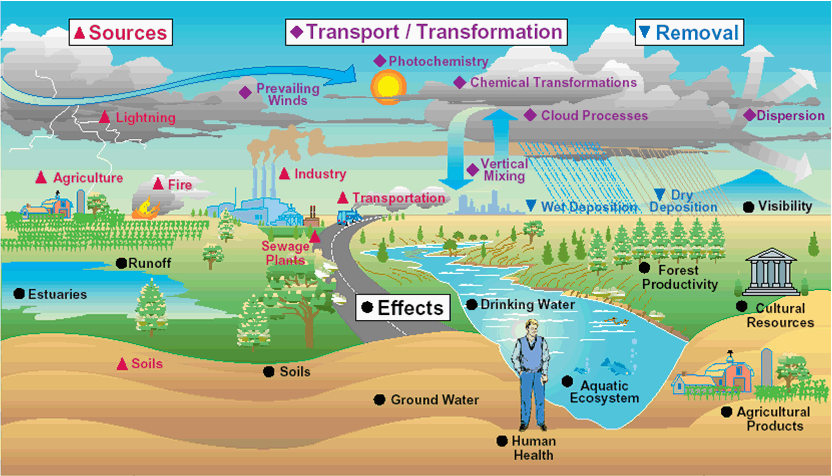
Air Pollution Pathways
Smog
Smog is a kind of air pollution; the word "smog" is a portmanteau ( a word
or morpheme that fuses two or more words or word parts to give a combined or
loaded meaning) of smoke and fog.
"Smog" refers to a noxious mixture of air pollutants that can often be seen
as a haze in the air. It often stays for an extended period of time over
densely populated cities or urban areas, such as London, New York, Los
Angeles, Mexico City, Houston, Toronto, Athens, Beijing andHong Kong.
A temperature inversion occurs when air close to the earth is cooler than
the air above it. Under these conditions the pollution cannot rise and be
dispersed. Cities surrounded by mountains also experience trapping of
pollution. Inversion can happen in any season. Winter inversions are likely
to cause particulate and cabon monoxide pollution. Summer inversions are
more likely to create smog.

Los Angeles Smog
Smog can make breathing more difficult -- even for healthy people -- and it
can make us more susceptible to cardio-respiratory diseases. Even healthy
young adults breathe less efficiently on days when the air is heavily
polluted, especially if exercising outdoors.

Beijing China air on a day after rain (left) and a sunny but smoggy day
(right) August 2005.
Photo taken by Bobak Ha'Eri
Particularly vulnerable to smog are people with heart or lung disease, the
elderly and small children. The two main ingredients in smog that affect our
health are ground-level ozone and fine airborne particles.
Ground-level Ozone
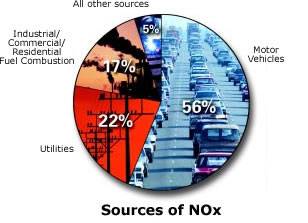 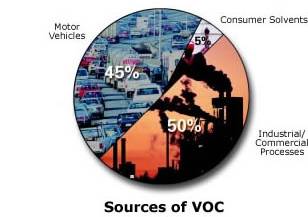
EPA Graphic
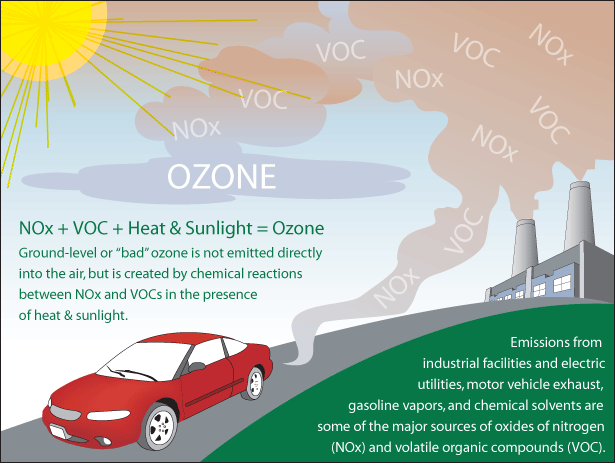
http://airnow.gov
Ground-level ozone
is a colorless and highly irritating gas that forms just above the earth's
surface. It is called a "secondary" pollutant because it is produced when two
primary pollutants react in sunlight and stagnant air. These two primary
pollutants are nitrogen oxides (NOx) and volatile organic compounds (VOC). NOx
and VOC come from natural sources as well as human activities.
NOx are
nitrogen-oxygen compounds that include the gases nitric oxide and nitrogen
dioxide, and are produced mostly by burning fossil fuels. VOC are
carbon-containing gases and vapors such as gasoline fumes (but excluding carbon
dioxide, carbon monoxide, methane, and chlorofluorocarbons).
Human activities are
responsible for the increases in ground-level ozone in recent years. About 95
per cent of nitrogen oxides from human activity come from the burning of coal,
gas and oil in motor vehicles, homes, industries and power plants. VOC come
mainly from fuel combustion and from the evaporation of liquid fuels and
solvents.
Ozone not only
affects human health, it can damage vegetation and decrease the productivity of
some crops. It can also injure flowers and shrubs and may contribute to forest
decline in some parts of Canada. Ozone can also damage synthetic materials,
cause cracks in rubber, accelerate fading of dyes, and speed deterioration of
some paints and coatings. As well, it damages cotton, acetate, nylon, polyester
and other textiles.
Airborne
Particles
Airborne particles
are microscopic and remain suspended in the air for some time. Particles can be
both primary pollutants and secondary pollutants, sent directly into the
atmosphere in the form of windblown dust and soil, sea salt spray, pollen and
spores. Secondary particles are formed through chemical reactions involving
nitrogen oxides, sulfur dioxide, VOCs and ammonia.
Particles give smog
its color and affect visibility. Depending on the type of particles, the air can
appear yellowish-brown, or even white. Like ozone, particles are believed to
have adverse effects on vegetation, and on various synthetic and natural
surfaces. .
Other Pollutants
in Smog
Nitrogen Dioxide (NO2
) is a principal member of the family of nitrogen oxides (NOx ). It is a toxic,
irritating gas that results from all combustion processes.
Sulphur dioxide (SO2)
is a colorless gas that smells like burnt matches. It can be chemically
transformed into acidic pollutants such as sulfuric acid and sulfates (sulfates
are a major component of fine particles). The main sources of airborne SO2
are coal-fired power generating stations and non-ferrous ore smelters. Sulfur
dioxide is also the main cause of acid rain, which can damage crops, forests and
whole ecosystems.
Carbon Monoxide (CO)
is a colorless, odorless and tasteless gas that comes primarily from automobile
emissions.
Acid Rain
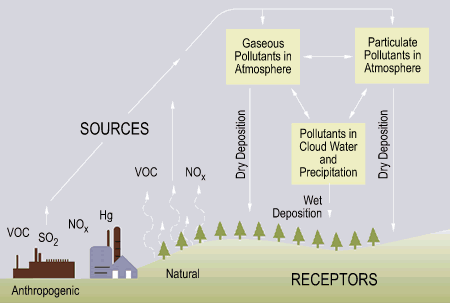
EPA Graphic
"Acid rain" is a
broad term referring to a mixture of wet and dry deposition (deposited material)
from the atmosphere containing higher than normal amounts of nitric and sulfuric
acids. The precursors, or chemical forerunners, of acid rain formation result
from both natural sources, such as volcanoes and decaying vegetation, and
man-made sources, primarily emissions of sulfur dioxide (SO2) and nitrogen
oxides (NOx) resulting from fossil fuel combustion. In the United States,
roughly 2/3 of all SO2 and 1/4 of all NOx come from electric power generation
that relies on burning fossil fuels, like coal. Acid rain occurs when these
gases react in the atmosphere with water, oxygen, and other chemicals to form
various acidic compounds. The result is a mild solution of sulfuric acid and
nitric acid. When sulfur dioxide and nitrogen oxides are released from power
plants and other sources, prevailing winds blow these compounds across state and
national borders, sometimes over hundreds of miles.
Wet Deposition
Wet deposition refers to acidic rain, fog, and snow. If the acid chemicals in
the air are blown into areas where the weather is wet, the acids can fall to the
ground in the form of rain, snow, fog, or mist. As this acidic water flows over
and through the ground, it affects a variety of plants and animals. The strength
of the effects depends on several factors, including how acidic the water is;
the chemistry and buffering capacity of the soils involved; and the types of
fish, trees, and other living things that rely on the water.
Dry Deposition
In areas where the weather is dry, the acid chemicals may become incorporated
into dust or smoke and fall to the ground through dry deposition, sticking to
the ground, buildings, homes, cars, and trees. Dry deposited gases and particles
can be washed from these surfaces by rainstorms, leading to increased runoff.
This runoff water makes the resulting mixture more acidic. About half of the
acidity in the atmosphere falls back to earth through dry deposition.
Acid rain causes
acidification of lakes and streams and contributes to the damage of trees at
high elevations (for example, red spruce trees above 2,000 feet) and many
sensitive forest soils. In addition, acid rain accelerates the decay of building
materials and paints, including irreplaceable buildings, statues, and sculptures
that are part of our nation's cultural heritage. Prior to falling to the earth,
sulfur dioxide (SO2) and nitrogen oxide (NOx) gases and their particulate matter
derivatives—sulfates and nitrates—contribute to visibility degradation and harm
public health.
Some of the problems
attributed to acid rain include:
-
Trees lose some
of the protection in their leaves, leaving them more at risk from frost and
diseases.
-
Tree roots may
also become stunted, so they can't take up as many nutrients.
-
Soils lose some
of their nutrients.
-
Increasing acid
levels may cause problems for aquatic animals and plants. Some fish may have
trouble breathing for example.
-
Acid rain may
dissolve the stonework and mortar of buildings causing structural problems
of buildings.
How Acid Rain
Harms Trees
Acid rain does not usually kill trees directly. Instead, it is more likely to
weaken trees by damaging their leaves, limiting the nutrients available to them,
or exposing them to toxic substances slowly released from the soil. Quite often,
injury or death of trees is a result of these effects of acid rain in
combination with one or more additional threats.

Acid rain, woods, Jizera Mountains, Czech Republic
Credit: Lovecz -Prague
Scientists know that
acidic water dissolves the nutrients and helpful minerals in the soil and then
washes them away before trees and other plants can use them to grow. At the same
time, acid rain causes the release of substances that are toxic to trees and
plants, such as aluminum, into the soil. Scientists believe that this
combination of loss of soil nutrients and increase of toxic aluminum may be one
way that acid rain harms trees. Such substances also wash away in the runoff and
are carried into streams, rivers, and lakes. More of these substances are
released from the soil when the rainfall is more acidic.
However, trees can
be damaged by acid rain even if the soil is well buffered. Forests in high
mountain regions often are exposed to greater amounts of acid than other forests
because they tend to be surrounded by acidic clouds and fog that are more acidic
than rainfall. Scientists believe that when leaves are frequently bathed in this
acid fog, essential nutrients in their leaves and needles are stripped away.
This loss of nutrients in their foliage makes trees more susceptible to damage
by other environmental factors, particularly cold winter weather.
Human Health
Acid rain looks,
feels, and tastes just like clean rain. The harm to people from acid rain is not
direct. Walking in acid rain, or even swimming in an acid lake, is no more
dangerous than walking or swimming in clean water. However, the pollutants that
cause acid rain—sulfur dioxide (SO2) and nitrogen oxides (NOx)—do damage human
health. These gases interact in the atmosphere to form fine sulfate and nitrate
particles that can be transported long distances by winds and inhaled deep into
people's lungs. Fine particles can also penetrate indoors. Many scientific
studies have identified a relationship between elevated levels of fine particles
and increased illness and premature death from heart and lung disorders, such as
asthma and bronchitis.
Based on health
concerns, SO2 and NOx have historically been regulated under the Clean Air Act,
including the Acid Rain Program. In the eastern U.S., sulfate aerosols make up
about 25 percent of fine particles. By lowering SO2 and NOx emissions from power
generation, the Acid Rain Program will reduce the levels of fine sulfate and
nitrate particles and so reduce the incidence and the severity of these health
problems. When fully implemented by the year 2010, the public health benefits of
the Acid Rain Program are estimated to be valued at $50 billion annually, due to
decreased mortality, hospital admissions, and emergency room visits.
Decreases in NOx
emissions are also expected to have a beneficial impact on human health by
reducing the nitrogen oxides available to react with volatile organic compounds
and form ozone. Ozone impacts on human health include a number of morbidity and
mortality risks associated with lung inflammation, including asthma and
emphysema.
Wet Deposition
Wet deposition
refers to acidic rain, fog, and snow. If the acid chemicals in the air are blown
into areas where the weather is wet, the acids can fall to the ground in the
form of rain, snow, fog, or mist. As this acidic water flows over and through
the ground, it affects a variety of plants and animals. The strength of the
effects depends on several factors, including how acidic the water is; the
chemistry and buffering capacity of the soils involved; and the types of fish,
trees, and other living things that rely on the water.
Dry Deposition
In areas where the
weather is dry, the acid chemicals may become incorporated into dust or smoke
and fall to the ground through dry deposition, sticking to the ground,
buildings, homes, cars, and trees. Dry deposited gases and particles can be
washed from these surfaces by rainstorms, leading to increased runoff. This
runoff water makes the resulting mixture more acidic. About half of the acidity
in the atmosphere falls back to earth through dry deposition.
The Greenhouse
Effect
The Greenhouse
Effect, also referred to as global warming, is generally believed to come from
the build up of carbon dioxide gas in the atmosphere. Carbon dioxide is produced
when fuels are burned. Plants convert carbon dioxide back to oxygen, but the
release of carbon dioxide from human activities is higher than the world's
plants can process. The situation is made worse since many of the earth's
forests are being removed, and plant life is being damaged by acid rain. Thus,
the amount of carbon dioxide in the air is continuing to increase. This buildup
acts like a blanket and traps heat close to the surface of our earth. Changes of
even a few degrees will affect us all through changes in the climate and even
the possibility that the polar ice caps may melt. (One of the consequences of
polar ice cap melting would be a rise in global sea level, resulting in
widespread coastal flooding.)
Ozone depletion
Ozone depletion is
another result of pollution. Chemicals released by our activities affect the
stratosphere , one of the atmospheric layers surrounding earth. The ozone layer
in the stratosphere protects the earth from harmful ultraviolet radiation from
the sun. Release of chlorofluorocarbons (CFC's) from aerosol cans, cooling
systems and refrigerator equipment removes some of the ozone, causing "holes";
to open up in this layer and allowing the radiation to reach the earth.
Ultraviolet radiation is known to cause skin cancer and has damaging effects on
plants and wildlife.
Travel and Air pollution
In The United States the use of SUVS (Suburban Utility Vehicles) is the
latest fad and passion. SUVS on average releases 5,600 pounds of CO2
into the atmosphere each year, double the amount of the average car
driven the same distance.
Air travel has become commonplace for vacationers, but not with out it's
unseen costs to the environment. A round trip flight from New York to
Los Angeles release as much as one automobile does in an entire year. On
a yearly basis all air travel releases 600 million tons of carbon
dioxide into the atmosphere.

1 round trip flight from NY to LA = 2,000 pounds of CO2
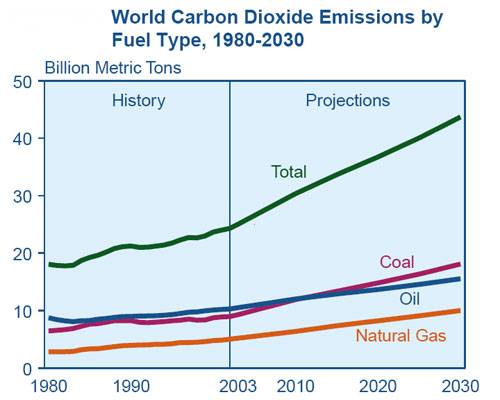
source: US Department of Energy
Global Air Pollution
Global air pollution continues to worsen do to the population growth and
scant environmental restrictions in many nations. It is becoming
increasingly hazardous to the health of The Earth.
There are six major outdoor air pollutants- ozone, particulate matter,
carbon monoxide, lead, nitrogen dioxide, and sulfur dioxide.
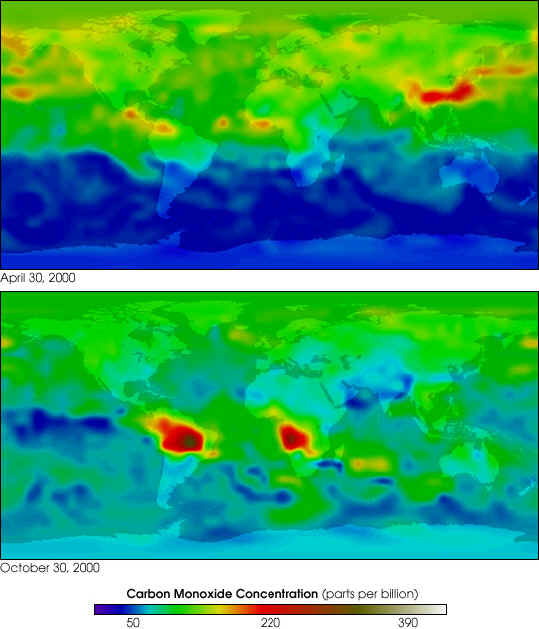
NASA GSFC Scientific Visualization Studio, based on data from MOPITT
(Canadian Space Agency and University of Toronto.) Satellite: Terra Sensor:
MOPITT
NASA's Terra spacecraft has assembled the most complete view ever of the
world's air pollution travelling through the atmosphere, across continents
and oceans. For the first time, policymakers and scientists now have a way
to identify the major sources of air pollution and to closely track where
the pollution goes, anywhere on Earth. The false colors in these images
represent levels of carbon monoxide in the lower atmosphere, ranging from
about 390 parts per billion (dark brown pixels), to 220 parts per billion
(red pixels), to 50 parts per billion (blue pixels). Carbon monoxide is a
gaseous byproduct from the burning of fossil fuels, in industry and
automobiles, as well as burning of forests and grasslands. Notice in the
April 30, 2000, image that levels of carbon monoxide are much higher in the
Northern Hemisphere, where human population and human industry is much
greater than in the Southern Hemisphere. However, in the October 30, 2000,
image notice the immense plumes of the gas emitted from forest and grassland
fires burning in South America and Southern Africa.
Indoor Air
Pollution
We usually
think of air pollution as being outdoors, but the air in your house or
office could also be polluted. Sources of indoor pollution include
Biological contaminants like mold and pollen Tobacco smoke Household
products and pesticides Gases such as radon and carbon monoxide Materials
used in the building such as asbestos, formaldehyde and lead
The levels of
pollutants in the air inside homes, schools, and other buildings can be
higher than the level of pollutants in the outdoor air. Indoor air
pollution comprises a mixture of contaminants penetrating from outdoors
and those generated indoors. In the last several years, the amount of
scientific evidence has indicated that the air within homes and other
buildings can be more seriously polluted than the outdoor air in even the
largest and most industrialized cities. Other research indicates that
people spend approximately 90 percent of their time indoors. In addition,
people who may be exposed to indoor air pollutants for the longest periods
of time are often those most susceptible to the effects of indoor
pollution. Such groups include the young, the elderly, and the chronically
ill, especially those suffering from respiratory or cardiovascular
disease.
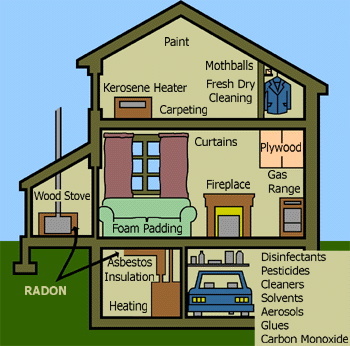
AIR POLLUTION SOURCES IN THE HOME
There are many sources of
indoor air pollution in homes. These sources of indoor air pollution
include combustion sources (oil, gas, kerosene, coal, wood, tobacco
products), building materials, wet or damp carpet, cabinetry or furniture
made of certain pressed wood products; household cleaning products,
central heating and cooling systems, humidification devices, and outdoor
sources such as radon, pesticides, and outdoor air pollution.
The relative importance of
any single source depends on how much of a given pollutant it emits and
how hazardous those emissions are. In some cases, factors such as how old
the source is and whether it is properly maintained are significant. For
example, an improperly adjusted gas stove can emit significantly more
carbon monoxide than one that is properly adjusted.
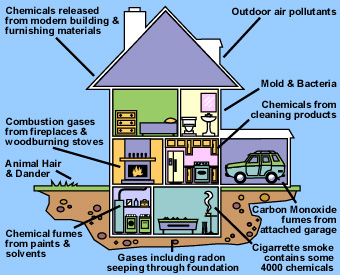
Radon and environmental
tobacco smoke (ETS) are the two indoor air pollutants of greatest concern
from a health perspective. Radon is a naturally occurring gas that
is odorless, colorless, and radioactive. Environmental tobacco smoke
(ETS) is the smoke emitted from the burning of a cigarette, pipe, or
cigar, and smoke inhaled by a smoker. It is a complex mix of more than
4,000 chemical compounds, containing many known or suspected carcinogens
and toxic agents, including particles, carbon monoxide, and formaldehyde.
More than three billion
people worldwide continue to depend on solid fuels, including biomass
fuels (wood, dung, agricultural residues) and coal, for their energy
needs.
Cooking and heating with
solid fuels on open fires or traditional stoves results in high levels of
indoor air pollution. Indoor smoke contains a range of health-damaging
pollutants, such as small particles and carbon monoxide, and particulate
pollution levels may be 20 times higher than accepted guideline values.
- Poor indoor air quality
can cause or contribute to the development of chronic respiratory
diseases such as asthma and hypersensitivity pneumonitis. In addition,
it can cause headaches, dry eyes, nasal congestion, nausea and fatigue.
People who already have respiratory diseases are at greater risk.
-
Biological pollutants, including molds, bacteria, viruses, pollen,
dust mites, and animal dander promote poor indoor air quality and may
be a major cause of days lost from work and school. In office
buildings, heating, cooling, and ventilation systems are frequent
sources of biological substances that are inhaled, leading to
breathing problems.
-
To help prevent growth of mold when humidity is high, make sure
bathrooms, kitchens and basements have good air circulation and are
cleaned often. The basement in particular may need a dehumidifier. And
remember, the water in the dehumidifier must be emptied and the
container cleaned often to prevent forming mildew.
-
An estimated one out of every 15 homes in the United States has radon
levels above 4pci/L, the U. S. Environmental Protection
Agency-recommended action level. Radon, a naturally occurring gas, can
enter the home through cracks in the foundation floor and walls,
drains, and other openings. Indoor radon exposure is estimated to be
the second leading cause of lung cancer. A recent report by the
National Research Council estimates that radon is responsible for
between 15,000 and 21,000 lung cancer deaths each year in the United
States.
-
Environmental tobacco smoke (ETS) also called "secondhand smoke," a
major indoor air pollutant, contains about 4,000 chemicals, including
200 known poisons, such as formaldehyde and carbon monoxide, as well
as 43 carcinogens.
-
ETS causes an estimated 3,000 lung cancer deaths and 35,000 to 50,000
heart disease deaths in non-smokers, as well as 150,000 to 300,000
cases of lower respiratory tract infections in children under 18
months of age each year.
-
Formaldehyde is a common chemical, found primarily in adhesive or
bonding agents for many materials found in households and offices,
including carpets, upholstery, particle board, and plywood paneling.
The release of formaldehyde into the air may cause health problems,
such as coughing; eye, nose, and throat irritation; skin rashes,
headaches, and dizziness.
-
Asbestos is the name given to a group of microscopic mineral fibers
that are flexible and durable and will not burn. Asbestos fibers are
light and small enough to remain airborne; they can be inhaled into
the lungs and can cause asbestosis (scarring of the lung tissue), lung
cancer and mesothelioma, a relatively uncommon cancer of the lining of
the lung or abdominal cavity.
-
Many asbestos products are found in the home, including roofing and
flooring materials, wall and pipe insulation, spackling compounds,
cement, coating materials, heating equipment, and acoustic insulation.
These products are a potential problem indoors only if the
asbestos-containing material is disturbed and becomes airborne, or
when it disintegrates with age.
-
Heating systems and other home appliances using gas, fuel, or wood,
can produce several combustion products, of which the most dangerous
are carbon monoxide (CO) and nitrogen dioxide (NO2). Fuel burning
stoves, furnaces, fireplaces, heaters, water heaters, and dryers are
all combustion appliances.
-
Carbon monoxide is an odorless, colorless gas that interferes with the
distribution of oxygen to the body. Depending on the amount inhaled,
this gas can impede coordination, worsen cardiovascular conditions,
and produce fatigue, headache, confusion, nausea, and dizziness. Very
high levels can cause death.
-
Nitrogen dioxide is a colorless, odorless gas that irritates the
mucous membranes in the eye, nose and throat and causes shortness of
breath after exposure to high concentrations. Prolonged exposure to
high levels of this gas can damage respiratory tissue and may lead to
chronic bronchitis.
-
Household cleaning agents, personal care products, pesticides, paints,
hobby products, and solvents may be sources of hundreds of potentially
harmful chemicals. Such components in many household and personal care
products can cause dizziness, nausea, allergic reactions,
eye/skin/respiratory tract irritation, and cancer.
Credit: EPA, CDC,Environment Canada, UNEP, NASA, Lawrence Berkley National
Laboratory, American Lung association
|

