|
Many chemical
compounds found in the Earth’s atmosphere act as “greenhouse gases.” These gases
allow sunlight to enter the atmosphere freely. When sunlight strikes the Earth’s
surface, some of it is reflected back towards space as infrared radiation
(heat). Greenhouse gases absorb this infrared radiation and trap the heat in the
atmosphere. Over time, the amount of energy sent from the sun to the Earth’s
surface should be about the same as the amount of energy radiated back into
space, leaving the temperature of the Earth’s surface roughly constant. Many
gases exhibit these “greenhouse” properties. Some of them occur in nature (water
vapor, carbon dioxide, methane, and nitrous oxide), while others are exclusively
human-made (like gases used for aerosols).
This natural
“carbon cycle” includes carbon dioxide used in plants during photosynthesis and
the exchange of carbon dioxide between the atmosphere and the oceans. The
primary natural processes that release CO2 into the atmosphere (sources) and
that remove CO2 from the atmosphere (sinks) are:
-
Animal and plant respiration, by which oxygen and nutrients are converted
into CO2 and energy, and plant photosynthesis by which CO2 is removed from
the atmosphere and stored as carbon in plant biomass
-
Ocean-atmosphere exchange, in which the oceans absorb and release CO2 at the
sea surface;
-
Volcanic eruptions, which release carbon from rocks deep in the Earth’s
crust
Naturally occurring greenhouse gases include water vapor, carbon dioxide,
methane, nitrous oxide, and ozone. Water vapor accounts for the largest
percentage of the greenhouse effect.
Earth's atmosphere is 78% nitrogen, 21% oxygen, and 1% other gases. Carbon
dioxide accounts for just 0.03 - 0.04%. Water vapor, varying in amount from 0 to
2%, carbon dioxide and some other minor gases present in the atmosphere absorb
some of the thermal radiation leaving the surface and emit radiation from much
higher and colder levels out to space. These active gases are known as
greenhouse gases because they act as a partial blanket for the thermal radiation
from the surface and enable it to be substantially warmer than it would
otherwise be, analogous to the effect of a greenhouse. This blanketing is known
as the natural greenhouse effect. Without the greenhouse gases, Earth's average
temperature would be roughly -20°C. 
The major greenhouse
gases are:
-
water vapor,
which causes about 36–70% of the greenhouse effect on Earth (not including
clouds)
-
carbon
dioxide, which causes 9–26%
-
methane,
which causes 4–9%
-
ozone,
which causes 3–7%.
-
It is not
possible to state that a certain gas causes a certain percentage of the
greenhouse effect, because the influences of the various gases are not
additive. (The higher ends of the ranges quoted are for the gas alone; the
lower ends, for the gas counting overlaps.)
-
Other
greenhouse gases include, but are not limited to, nitrous oxide, sulfur
hexafluoride, hydrofluorocarbons, perfluorocarbons and chlorofluorocarbons.
Certain human
activities, however, add to the levels of most of these naturally occurring
gases by the following processes:
-
Carbon dioxide is released to the atmosphere when solid waste,
fossil fuels (oil, natural gas, and coal), and wood and wood products are
burned.
-
Methane is emitted during the production and transport of coal,
natural gas, and oil. Methane emissions also result from the decomposition
of organic wastes in municipal solid waste landfills, and the raising of
livestock.
-
Nitrous oxide is emitted during agricultural and industrial
activities, as well as during combustion of solid waste and fossil fuels.
Very powerful greenhouse gases that are not naturally occurring include
hydrofluorocarbons
(HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6),
which are generated in a variety of industrial processes.
Each greenhouse gas differs in its ability to absorb heat in the atmosphere.
HFCs and PFCs are the most heat-absorbent. Methane traps over 21 times more heat
per molecule than carbon dioxide, and nitrous oxide absorbs 270 times more heat
per molecule than carbon dioxide. Often, estimates of greenhouse gas emissions
are presented in units of millions of metric tons of carbon equivalents (MMTCE),
which weights each gas by its GWP value, or Global Warming Potential.
The principal greenhouse gases are:
-
Water vapor (H2O)
-
Carbon dioxide (also known as CO2 )
-
Methane (CH4)
-
Nitrous oxide (N2O)
-
Chlorofluorocarbons (CFCs)
-
Ozone (O3 )
The amount of CO2 released into the atmosphere in the next 30 years is expected
to double or triple. The number of cars in operation around the world will
double by the year 2030.
Many chemical compounds present
in Earth's atmosphere behave as 'greenhouse gases'. These are gases which allow
direct sunlight (relative shortwave energy) to reach the Earth's surface
unimpeded. As the shortwave energy (that in the visible and ultraviolet portion
of the spectra) heats the surface, longer-wave (infrared) energy (heat) is
reradiated to the atmosphere. Greenhouse gases absorb this energy, thereby
allowing less heat to escape back to space, and 'trapping' it in the lower
atmosphere. Many greenhouse gases occur naturally in the atmosphere, such as
carbon dioxide, methane, water vapor, and nitrous oxide, while others are
synthetic. Those that are man-made include the chlorofluorocarbons (CFCs),
hydrofluorocarbons (HFCs) and Perfluorocarbons (PFCs), as well as sulfur
hexafluoride (SF6). Atmospheric concentrations of both the natural
and man-made gases have been rising over the last few centuries due to the
industrial revolution. As the global population has increased and our reliance
on fossil fuels (such as coal, oil and natural gas) has been firmly solidified,
so emissions of these gases have risen. While gases such as carbon dioxide occur
naturally in the atmosphere, through our interference with the carbon cycle
(through burning forest lands, or mining and burning coal), we artificially move
carbon from solid storage to its gaseous state, thereby increasing atmospheric
concentrations.
Water Vapor
Water Vapor is the most abundant
greenhouse gas in the atmosphere, which is why it is addressed here first.
However, changes in its concentration is also considered to be a result of
climate feedbacks related to the warming of the atmosphere rather than a
direct result of industrialization. The feedback loop in which water is involved
is critically important to projecting future climate change, but as yet is still
fairly poorly measured and understood.
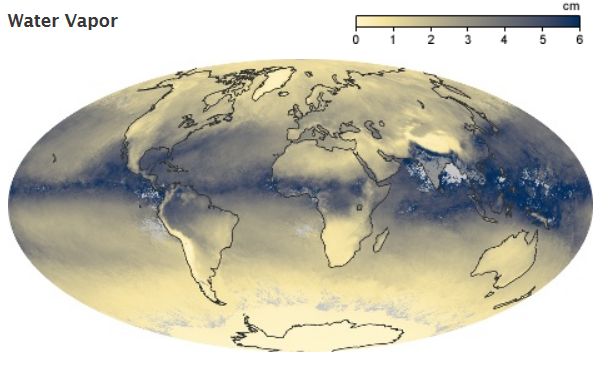
As the temperature of the
atmosphere rises, more water is evaporated from ground storage (rivers, oceans,
reservoirs, soil). Because the air is warmer, the relative humidity can be
higher (in essence, the air is able to 'hold' more water when its warmer),
leading to more water vapor in the atmosphere. As a greenhouse gas, the
higher concentration of water vapor is then able to absorb more thermal IR
energy radiated from the Earth, thus further warming the atmosphere. The warmer
atmosphere can then hold more water vapor and so on and so on. This is referred
to as a 'positive feedback loop'. However, huge scientific uncertainty exists in
defining the extent and importance of this feedback loop. As water vapor
increases in the atmosphere, more of it will eventually also condense into
clouds, which are more able to reflect incoming solar radiation (thus allowing
less energy to reach the Earth's surface and heat it up). The future monitoring
of atmospheric processes involving water vapor will be critical to fully
understand the feedbacks in the climate system leading to global climate change.
As yet, though the basics of the hydrological cycle are fairly well understood,
we have very little comprehension of the complexity of the feedback loops. Also,
while we have good atmospheric measurements of other key greenhouse gases such
as carbon dioxide and methane, we have poor measurements of global water vapor,
so it is not certain by how much atmospheric concentrations have risen in recent
decades or centuries, though satellite measurements, combined with balloon data
and some in-situ ground measurements indicate generally positive trends in
global water vapor.
Carbon
Dioxide
The natural production and
absorption of carbon dioxide (CO2) is achieved through the
terrestrial biosphere and the ocean. However, humankind has altered the natural
carbon cycle by burning coal, oil, natural gas and wood and since the industrial
revolution began in the mid 1700s, each of these actvities has increased in
scale and distribution. Carbon dioxide was the first greenhouse gas demonstrated
to be increasing in atmospheric concentration with the first conclusive
measurements being made in the last half of the 20th century. Prior to the
industrial revolution, concentrations were fairly stable at 280ppm. Today, they
are around 370ppm, an increase of well over 30%. The atmospheric concentration
has a marked seasonal oscillation that is mostly due to the greater extent of
landmass in the northern hemisphere (NH) and its vegetation. A greater drawdown
of CO2 occurs in the NH spring and summer as plants convert CO2
to plant material through photosynthesis. It is then released again in the fall
and winter as the plants decompose.
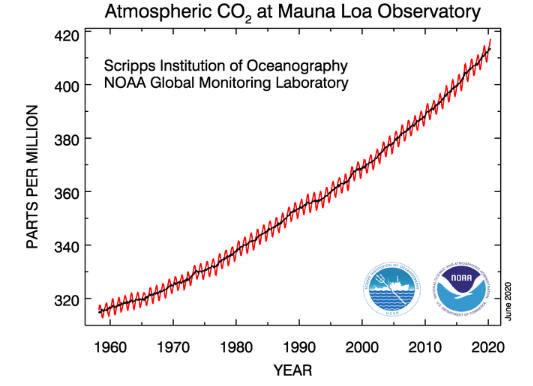
The graph shows recent monthly mean carbon dioxide measured at Mauna Loa
Observatory, Hawaii
Methane
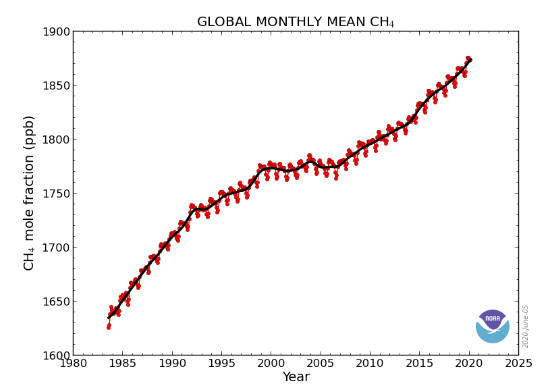
Methane is an extrememly
effective absorber of radiation, though its atmospheric concentration is less
than CO2 and its lifetime in the atmosphere is brief (10-12 years),
compared to some other greenhouse gases (such as CO2, N2O,
CFCs). Methane(CH4) has both natural and anthropogenic sources. It is
released as part of the biological processes in low oxygen environments, such as
in swamplands or in rice production (at the roots of the plants). Over the last
50 years, human activities such as growing rice, raising cattle, using natural
gas and mining coal have added to the atmospheric concentration of methane.
Tropospheric
Ozone
Ultraviolet radiation and oxygen
interact to form ozone in the stratosphere. Existing in a broad band, commonly
called the 'ozone layer', a small fraction of this ozone naturally descends to
the surface of the Earth. However, during the 20th century, this tropospheric
ozone has been supplemented by ozone created by human processes. The exhaust
emissions from automobiles and pollution from factories (as well as burning
vegetation) leads to greater concentrations of carbon and nitrogen molecules in
the lower atmosphere which, when it they are acted on by sunlight, produce
ozone. Consequently, ozone has higher concentrations in and around cities than
in sparsely populated areas, though there is some transport of ozone downwind of
major urban areas. Ozone is an important contributor to photochemical smog.
Though the lifetime of ozone is short, and is therefore not well-mixed through
the atmosphere, there is a general band of higher ozone concentration during NH
spring and summer between 30?N and 50?N resulting from the higher urbanization
and industrial activity in this band. Concentrations of ozone have risen by
around 30% since the pre-industrial era, and is now considered by the IPCC to be
the third most important greenhouse gas after carbon dioxide and methane. An
additional complication of ozone is that it also interacts with and is modulated
by concentrations of methane.
Nitrous
Oxide
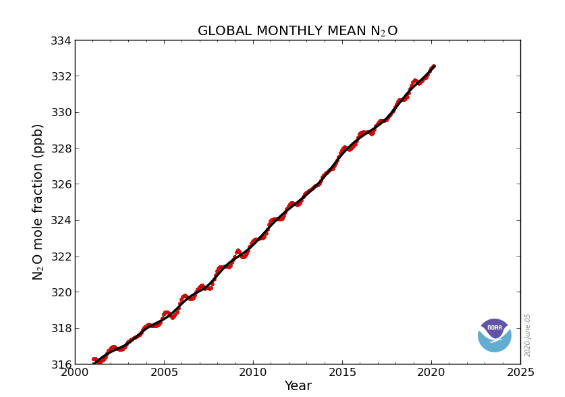
Concentrations of nitrous oxide
also began to rise at the beginning of the industrial revolution and is
understood to be produced by microbial processes in soil and water, including
those reactions which occur in fertilizer containing nitrogen. Increasing use of
these fertilizers has been made over the last century. and in addition to
agricultural sources for the gas, some industrial processes (fossil fuel-fired
power plants, nylon production, nitric acid production and vehicle emissions)
also contribute to its atmospheric load.
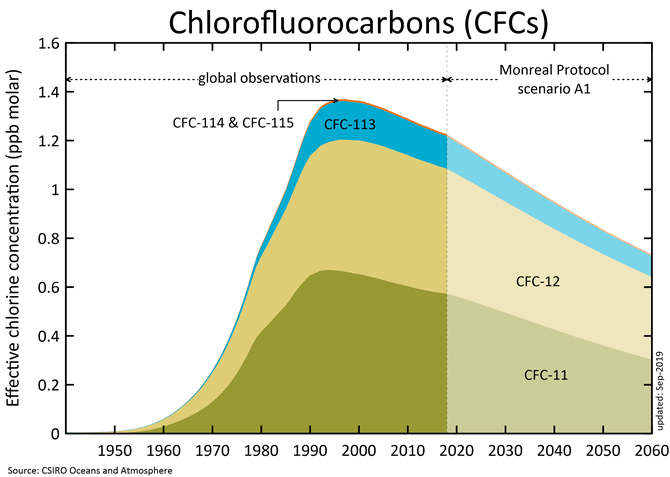
CFCs
etc.

CFCs (chlorofluorocarbons) have
no natural source, but were entirely synthesized for such diverse uses as
refrigerants, aerosol propellants and cleaning solvents. Their creation was in
1928 and since then concentrations of CFCs in the atmosphere have been rising.
Due to the discovery that they are able to destroy stratospheric ozone, a global
effort to halt their production was undertaken and was extremely successful. So
much so that levels of the major CFCs are now remaining level or declining.
However, their long atmospheric lifetimes determine that some concentration of
the CFCs will remain in the atmosphere for over 100 years. Since they are also
greenhouse gas, along with such other long-lived synthesized gases as CF4
(carbontatrafuoride), SF6 (sulfurhexafluoride), they are of concern.
Another set of synthesized compounds called HFCs (hydrofluorcarbons) are also
greenhouse gases, though they are less stable in the atmosphere and therefore
have a shorter lifetime and less of an impact as a greenhouse gas.
Carbon Monoxide and other
reactive gases
Carbon monoxide (CO) is not
considered a direct greenhouse gas, mostly because it does not absorb
terrestrial thermal IR energy strongly enough. However, CO is able to modulate
the production of methane and tropospheric ozone. The Northern Hemisphere
contains about twice as much CO as the Southern Hemisphere because as much as
half of the global burden of CO is derived from human activity, which is
predominantly located in the NH. Due to the spatial variability of CO, it is
difficult to ascertain global concentrations, however, it appears as though they
were generally increasing until the late 1980s, and have since begun to decline
somewhat. One possible explanation is the reduction in vehicle emissions of CO
since greater use of catalytic converters has been made.
Volatile Organic Compounds (VOCs)
also have a small direct impact as greenhouse gases, as well being involved in
chemical processes which modulate ozone production. VOCs include non-methane
hydrocarbons (NMHC), and oxygenated NMHCs (eg. alcohols and organic acids), and
their largest source is natural emissions from vegetation. However, there are
some anthropogenic sources such as vehicle emissions, fuel production and
biomass burning. Though measurement of VOCs is extremely difficult, it is
expected that most anthropogenic emissions of these compounds have increased in
recent decades.
U.S. Greenhouse
Gas Emissions by Gas
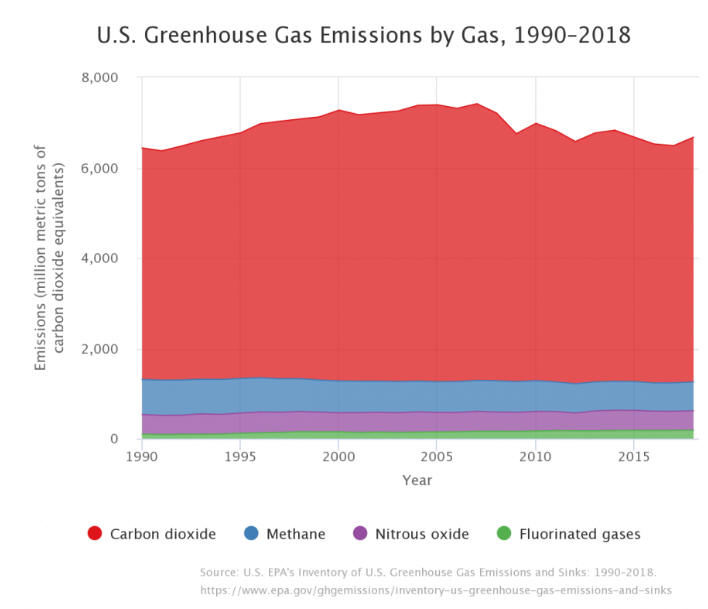
CO2 Emissions
from Fossil Fuel Combustion by Sector and Fuel Type
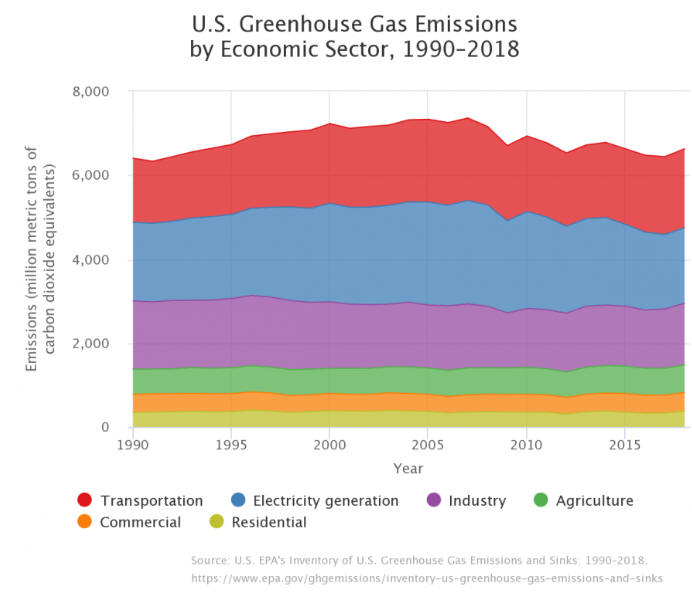
Source: EPA Greenhouse
Gas Inventory Report
The Driving
Force Of Greenhouse Gas Increase Is Population Growth
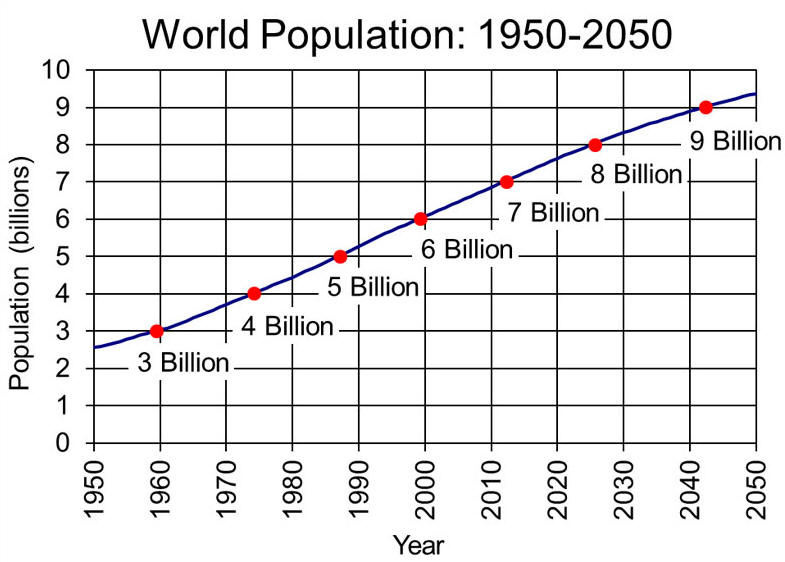
Between 1960 and 1999, Earth's population doubled from three billion to
six billion people. When population growth is coupled with shortsighted
planning and excessive consumption of resources, problems multiply.
Human pressure on the environment is a product of three factors:
population, consumption, and technology.
Population is the total number of people, consumption is the amount of
resources each person consumes, and technology is how these resources are
used and how much waste is produced for each unit of consumption.
We have transformed approximately half of The Earth's surface for our own
uses, with widespread impacts on the planet's forests, oceans,
freshwater, and atmosphere.
Credit: GRACE Goddard Space Flight
Center NASA, UNEP, EPA, Woods Hole Oceanographic Institute, NOAA, University of
Colorado, CIA, U.S. Department of Energy
|

