|
||||||||||||
|
|
|
Hydraulic Fracturing
A Chesapeake Energy natural-gas well site near Burlington, Pa.
Natural gas plays a key role in our nation’s clean energy future. The U.S. has vast reserves of natural gas that are commercially viable as a result of advances in horizontal drilling and hydraulic fracturing technologies enabling greater access to gas in shale formations. Responsible development of America's shale gas resources offers important economic, energy security, and environmental benefits.
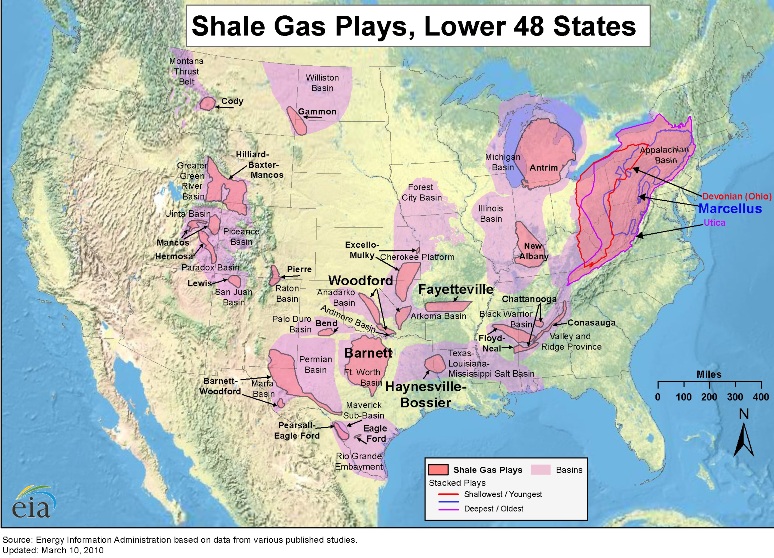
Although we’ve known for many years that natural gas was trapped in hard dense deposits of shale formed from ancient sea basins millions of years ago, we did not have the technology to access these resources economically until recently. As a result, previously uneconomic natural gas resources are now available for exploration and development. In the last five years, natural gas reserves grew 30 percent and in the last few years alone we have increased onshore natural gas production by more than 20 percent – an accomplishment that most energy experts thought impossible a few years ago. Shale gas “plays” are found throughout the Mountain West, the South and throughout the Northeast’s Appalachian Basin. These plays are geographic areas where companies are actively looking for natural gas in shale rock. The Barnett core in Texas, for example, is 5,000 square miles and provides 6 percent of U.S. natural gas. The Marcellus fairway that blankets Pennsylvania, New York, Ohio and West Virginia covers ten times the square miles of the Barnett, but has only recently started to be developed.
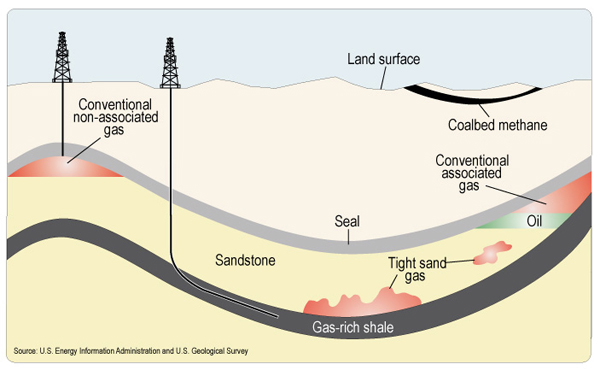
What is shale gas and why is it important?Shale gas refers to natural gas that is trapped within shale formations. Shales are fine-grained sedimentary rocks that can be rich sources of petroleum and natural gas. Over the past decade, the combination of horizontal drilling and hydraulic fracturing has allowed access to large volumes of shale gas that were previously uneconomical to produce. The production of natural gas from shale formations has rejuvenated the natural gas industry in the United States.
Does the U.S. Have Abundant Shale Gas Resources?Of the natural gas consumed in the United States in 2009, 87% was produced domestically; thus, the supply of natural gas is not as dependent on foreign producers as is the supply of crude oil, and the delivery system is less subject to interruption. The availability of large quantities of shale gas will further allow the United States to consume a predominantly domestic supply of gas.
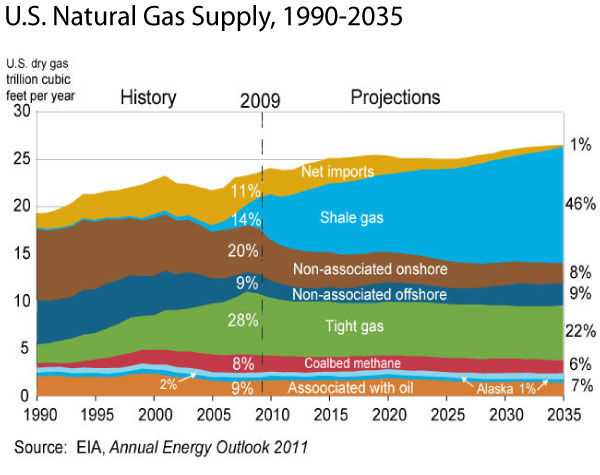
According to the EIA Annual Energy Outlook 2011, the United States possesses 2,543 trillion cubic feet (Tcf) of potential natural gas resources. Natural gas from shale resources, considered uneconomical just a few years ago, accounts for 862 Tcf of this resource estimate, more than double the estimate published last year. At the 2010 rate of U.S. consumption (about 24.1 Tcf per year), 2,543 Tcf of natural gas is enough to supply over 100 years of use. Shale gas resource and production estimates increased significantly between the 2010 and 2011 Outlook reports and are likely to increase further in the future. Where is Shale Gas Found?Shale gas is found in shale "plays," which are shale formations containing significant accumulations of natural gas and which share similar geologic and geographic properties. A decade of production has come from the Barnett Shale play in Texas. Experience and information gained from developing the Barnett Shale have improved the efficiency of shale gas development around the country. Another important play is the Marcellus Shale in the eastern United States. Surveyors and geologists identify suitable well locations in areas with potential for economical gas production by using both surface-level observation techniques and computer-generated maps of the subsurface.
Global Shale Plays
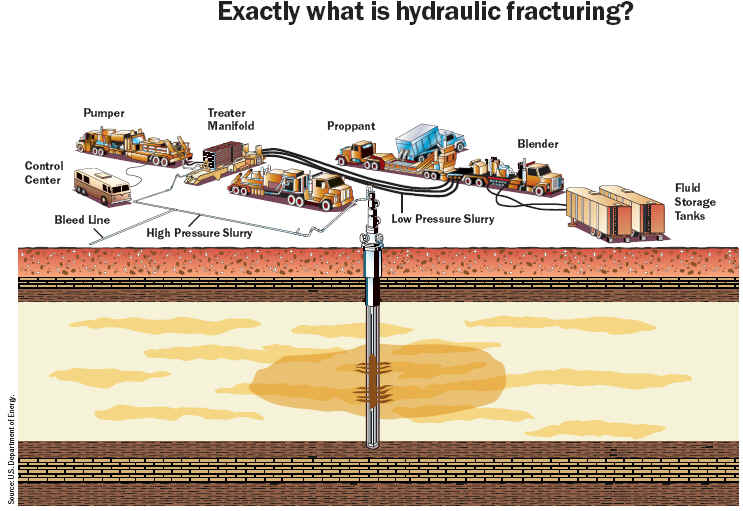
Hydraulic fracturing is a technology that was developed in the 1940s and has since helped produce more than 600 trillion cubic feet of natural gas and 7 billion barrels of oil.
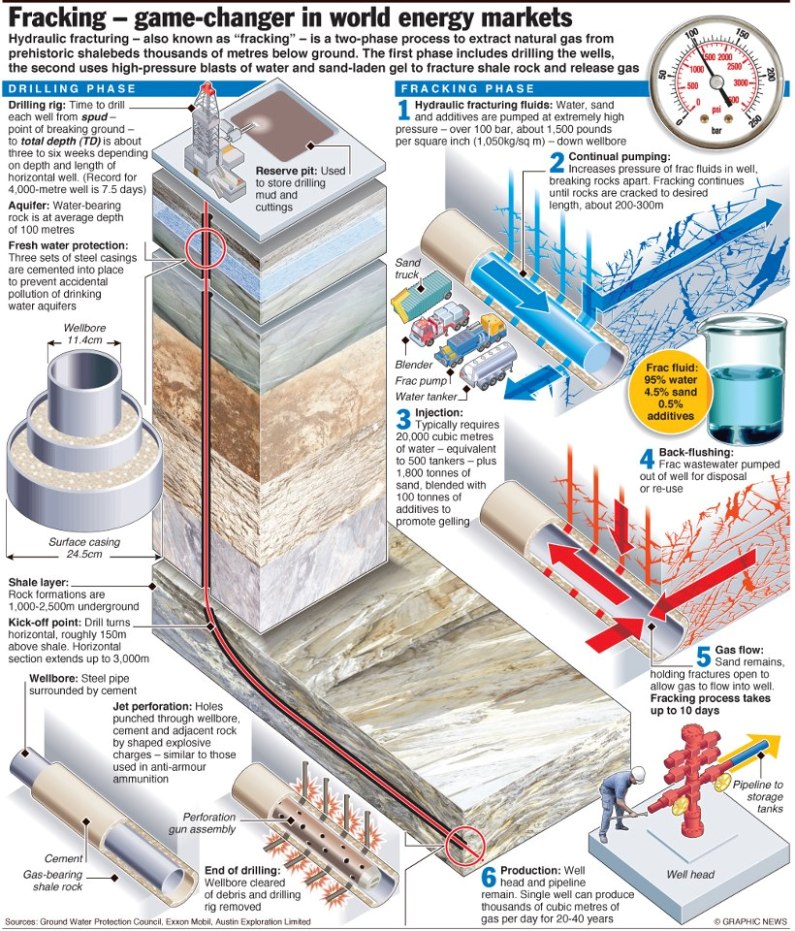
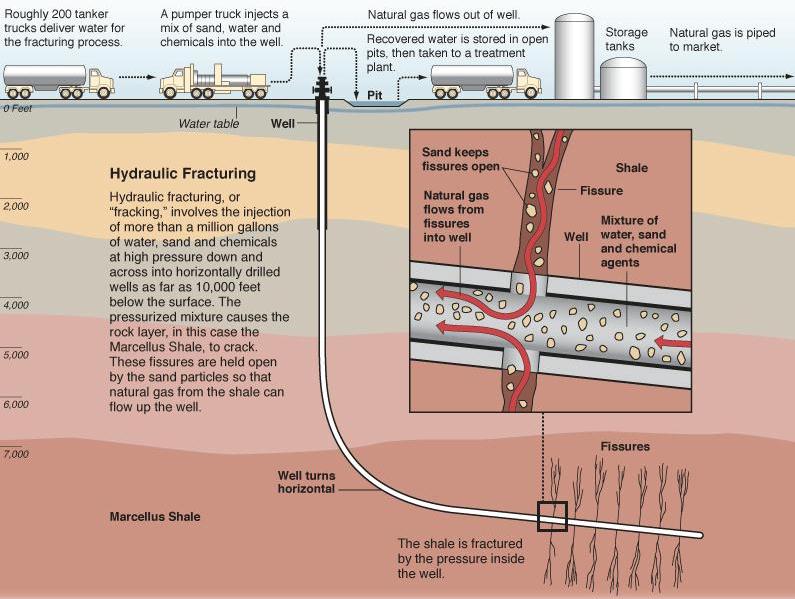
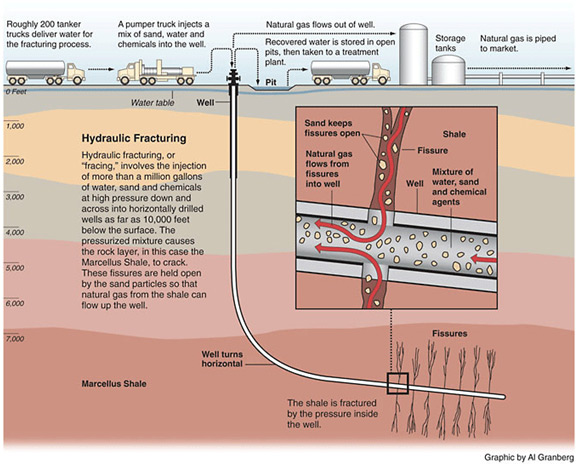
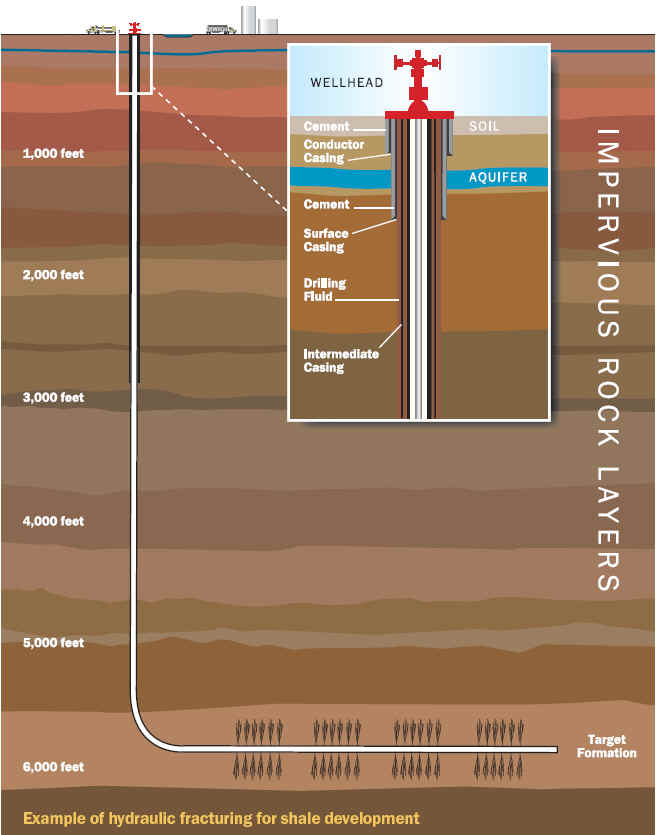
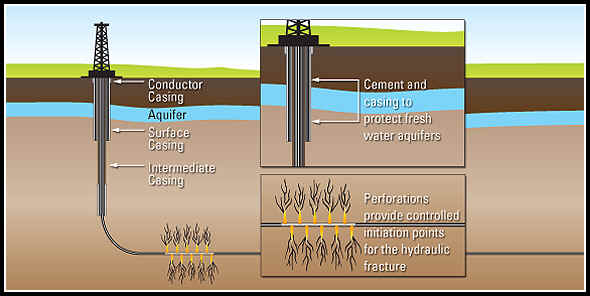
Hydraulic fracturing (commonly called “fracking” or “hydrofracking”) is a technique in which water, chemicals, and sand are pumped into the well to unlock the hydrocarbons trapped in shale formations by opening cracks (fractures) in the rock and allowing natural gas to flow from the shale into the well. When used in conjunction with horizontal drilling, hydraulic fracturing enables gas producers to extract shale gas at reasonable cost. Without these techniques, natural gas does not flow to the well rapidly, and commercial quantities cannot be produced from shale. How is Shale Gas Production Different from Conventional Gas Production?Conventional gas reservoirs are created when natural gas migrates toward the Earth's surface from an organic-rich source formation into highly permeable reservoir rock, where it is trapped by an overlying layer of impermeable rock. In contrast, shale gas resources form within the organic-rich shale source rock. The low permeability of the shale greatly inhibits the gas from migrating to more permeable reservoir rocks. Without horizontal drilling and hydraulic fracturing, shale gas production would not be economically feasible because the natural gas would not flow from the formation at high enough rates to justify the cost of drilling. The process of bringing a well to completion is generally short-lived, taking a few months for a single well, after which the well can be in production for 20 to 40 years. The process for a single horizontal well typically includes four to eight weeks to prepare the site for drilling, four or five weeks of rig work, including casing and cementing and moving all associated auxiliary equipment off the well site before fracturing operations commence, and two to five days for the entire multi-stage fracturing operation.
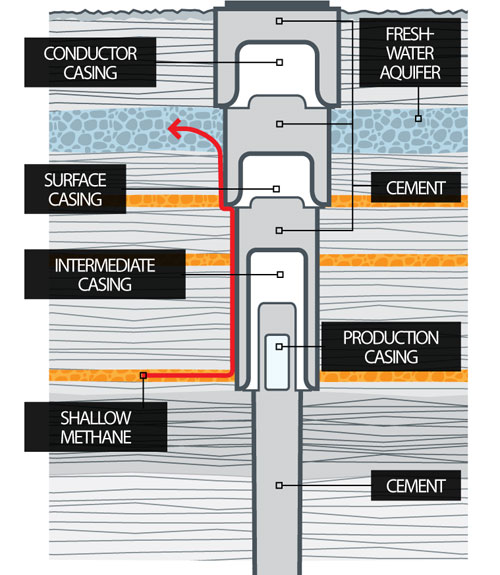
Typically, steel pipe known as surface casing is cemented into place at the uppermost portion of a well for the explicit purpose of protecting the groundwater. The depth of the surface casing is generally determined based on groundwater protection, among other factors. As the well is drilled deeper, additional casing is installed to isolate the formation(s) from which oil or natural gas is to be produced, which further protects groundwater from the producing formations in the well. Casing and cementing are critical parts of the well construction that not only protect any water zones but are also important to successful oil or natural gas production from hydrocarbon bearing zones. Water is an essential component of deep shale gas development during both the drilling and hydraulic fracturing, or fracking, processes. On the average 5 million gallons of water is needed to drill and fracture a typical deep shale gas well is equivalent to the amount of water consumed by:
What Are the Environmental Issues Associated with Shale Gas?
Natural gas is cleaner-burning than coal or oil. The combustion of natural gas emits significantly lower levels of carbon dioxide (CO2), nitrogen oxides, and sulfur dioxide than does the combustion of coal or oil. When used in efficient combined-cycle power plants, natural gas combustion can emit less than half as much CO2 as coal combustion, per unit of electricity output. However, there are some potential environmental concerns that are also associated with the production of shale gas. The fracturing of wells requires large amounts of water. In some areas of the country, significant use of water for shale gas production may affect the availability of water for other uses, and can affect aquatic habitats. Second, if mismanaged, hydraulic fracturing fluid — which may contain potentially hazardous chemicals — can be released by spills, leaks, or various other exposure pathways. Any such releases can contaminate surrounding areas.
Finally, fracturing also produces large amounts of wastewater, which may contain dissolved chemicals and other contaminants that require treatment before disposal or reuse. Because of the quantities of water used and the complexities inherent in treating some of the wastewater components, treatment and disposal is an important and challenging issue.
Credit: Energy Information Administration, U.S. Department of Energy,CIA, American Gas Association,Chesapeake Energy,ENCANA |