|
||||||||||||
|
|
|
Sea Ice
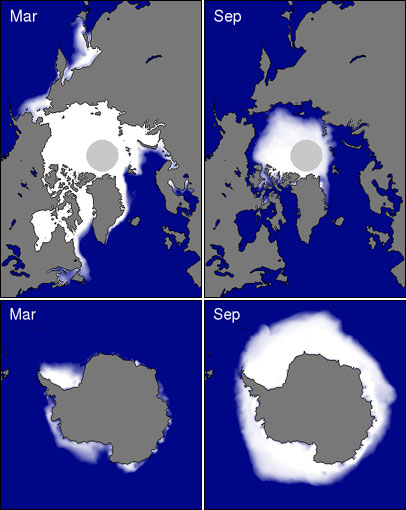
Sea ice is frozen seawater that floats on the ocean surface. Blanketing millions of square kilometers, sea ice forms and melts with the polar seasons, affecting both human activity and biological habitat. In the Arctic, some sea ice persists year after year, whereas almost all Southern Ocean or Antarctic sea ice is "seasonal ice," meaning it melts away and reforms annually. While both Arctic and Antarctic ice are of vital importance to the marine mammals and birds for which they are habitats, sea ice in the Arctic appears to play a more crucial role in regulating climate. Because they are composed of ice originating from glaciers, icebergs are not considered sea ice
The Importance of Sea IceSea ice has a profound influence on the polar physical environment, including ocean circulation, weather, and regional climate. As ice crystals form, they expel salt, which increases the salinity of the underlying ocean waters. This cold, salty water is dense, and it can sink deep to the ocean floor, where it flows back toward the equator. The sea ice layer also restricts wind and wave action near coastlines, lessening coastal erosion and protecting ice shelves. And sea ice creates an insulating cap across the ocean surface, which reduces evaporation and prevents heat loss to the atmosphere from the ocean surface. As a result, ice-covered areas are colder and drier than they would be without ice. Sea ice also has a fundamental role in polar ecosystems. When sea ice melts in the summer, it releases nutrients into the water, which stimulate the growth of phytoplankton, which are the base of the marine food web. As the ice melts, it exposes ocean water to sunlight, spurring photosynthesis in phytoplankton. When ice freezes, the underlying water gets saltier and sinks, mixing the water column and bringing nutrients to the surface. The ice itself is habitat for animals such as seals, Arctic foxes, polar bears, and penguins. Sea ice’s influence on the Earth is not just regional; it’s global. The white surface of sea ice reflects far more sunlight back to space than ocean water does. (In scientific terms, ice has a high albedo.) Once sea ice begins to melt, a self-reinforcing cycle often begins. As more ice melts and exposes more dark water, the water absorbs more sunlight. The sun-warmed water then melts more ice. Over several years, this positive feedback cycle (the “ice-albedo feedback”) can influence global climate. Sea ice plays many important roles in the Earth system, but influencing sea level is not one of them. Because it is already floating on the ocean surface, sea ice is already displacing its own weight. Melting sea ice won’t raise ocean level any more than melting ice cubes will cause a glass of iced tea to overflow.
The Sea Ice Life Cycle
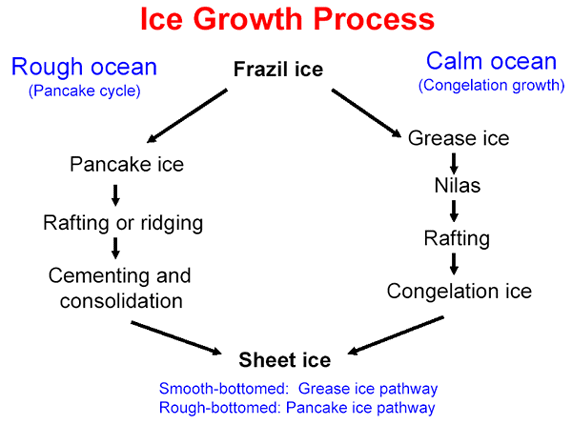
When seawater begins to freeze, it forms tiny crystals just millimeters wide, called frazil. How the crystals coalesce into larger masses of ice depends on whether the seas are calm or rough. In calm seas, the crystals form thin sheets of ice, nilas, so smooth they have an oily or greasy appearance. These wafer-thin sheets of ice slide over each other forming rafts of thicker ice. In rough seas, ice crystals converge into slushy pancakes. These pancakes can slide over each other to form smooth rafts, or they can collide into each other, creating ridges on the surface and keels on the bottom.
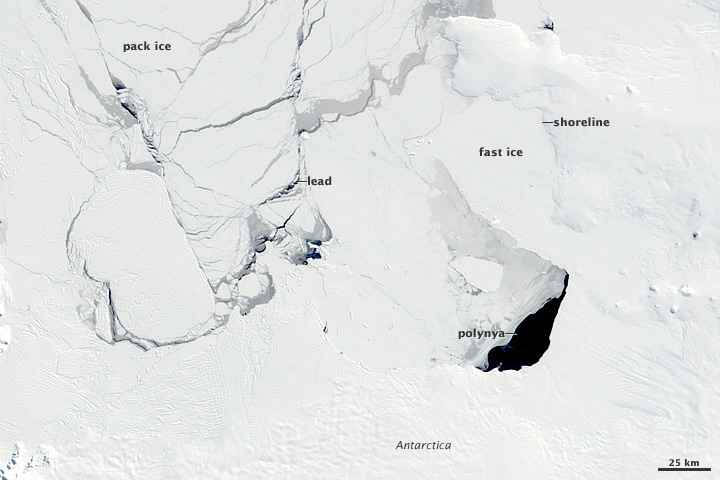
Sea ice begins as thin sheets of smooth nilas in calm water (top) or disks of pancake ice in choppy water (2nd from top). Individual pieces pile up on top of one another to form rafts and eventually solidify (3rd from top). Over time, large sheets of ice collide, forming thick pressure ridges along the margins (bottom). (Nilas, pancake, and ice raft photographs courtesy Don Perovich, Cold Regions Research and Engineering Laboratory. Pressure ridge photograph courtesy Ted Scambos, National Snow and Ice Data Center.) Some sea ice is fast ice that holds fast to a coastline or the sea floor, and some sea ice is pack ice that drifts with winds and currents. Because pack ice is dynamic, pieces of ice can collide and form much thicker ice. Leads—narrow, linear openings in the ice ranging in size from meters to kilometers—continually form and disappear. Larger and more persistent openings, polynyas, are sustained by upwelling currents of warm water or steady winds that blow the sea ice away from a spot as quickly as it forms. Polynyas often occur along coastlines where offshore winds maintain their presence.
Fast ice is anchored to the shore or the sea bottom, while pack ice floats freely. As it drifts, leads continually open and close between ice floes. Persistent openings, polynyas, are maintained by strong winds or ocean currents. (NASA satellite image courtesy Jacques Descloitres, MODIS Rapid Response Team.) As the water and air temperatures rise each summer, some sea ice melts. Because of differences in geography and climate, it’s normal for Antarctic sea ice to melt more completely in the summer than Arctic sea ice. Ice that escapes summer melting may last for years, often growing to a thickness of 2 to 4 meters (roughly 6.5 to 13 feet) or more in the Arctic. For ice to thicken, the ocean must lose heat to the atmosphere. But the ice insulates the ocean like a blanket. Eventually, the ice gets so thick that no more heat can escape. Once the ice reaches this thickness—3 to 4 meters (10 to 13 feet)—further thickening isn’t possible except through collisions and ridge-building. Ice that survives the summer melt season is called multi-year ice. Multi-year ice increasingly loses salt and hardens each year it survives the summer melt. In contrast to multi-year ice, first-year ice—ice that has grown just since the previous summer—is thinner, saltier, and more prone to melt in the subsequent summer.
Salinity and Brine Salinity is a measure of the concentration of dissolved salts in water. Until recently, a common way to define salinity values has been parts per thousand (ppt), or kilograms of salt in 1,000 kilograms of water. Today, salinity is usually described in practical salinity units (psu), a more accurate but more complex definition. Nonetheless, values of salinity in ppt and psu are nearly equivalent. The average salinity of the ocean typically varies from 32 to 37 psu, but in polar regions, it may be less than 30 psu. Sodium chloride (table salt) is the most abundant of the many salts found in the ocean. Fresh water freezes at 0 degrees Celsius (32 degrees Fahrenheit), but the freezing point of sea water varies. For every 5 ppt increase in salinity, the freezing point decreases by 0.28 degrees Celsius (0.5 degrees Fahrenheit); thus, in polar regions with an ocean salinity of 35 ppt, the water begins to freeze at -1.8 degrees Celsius (28.8 degrees Fahrenheit).
Salt plays an important role in ocean circulation. In cold, polar regions, changes in salinity affect ocean density more than changes in temperature. When salt is ejected into the ocean as sea ice forms, the water's salinity increases. Because salt water is heavier, the density of the water increases and the water sinks. The exchange of salt between sea ice and the ocean influences ocean circulation across hundreds of kilometers.
,NSIDC |